By Dennis Ford, Founder & CEO, Life Science Nation (LSN)
 Life Science Nation (LSN) has established a global partnering platform connecting scientist-entrepreneurs and fundraising CEOs with capital, licensing, and product collaboration partners. Along the way, LSN has gained valuable insights into what makes a strong match for our buy-side partners.
Life Science Nation (LSN) has established a global partnering platform connecting scientist-entrepreneurs and fundraising CEOs with capital, licensing, and product collaboration partners. Along the way, LSN has gained valuable insights into what makes a strong match for our buy-side partners.
Through discussions with hundreds of investors, I’ve explored how their pursuit of technology assets balances with the inherent risks of taking on unproven ventures. The answer is nuanced but typically revolves around a company’s key attributes: the science, the management team, milestones achieved, and the data proving the technology’s trajectory.
Similarly, I’ve developed a personal framework for evaluating companies referred to LSN through regional tech hubs, incubators, accelerators, and national or state life science agencies. Here are the qualities I prioritize:
The Founding Scientist
A founding scientist with a deep history in the science and technology at the heart of the company is invaluable. Many “new” technologies are refinements of decades-long research. When the original scientist remains involved—whether as a CTO, advisor, or board member—it helps de-risk the opportunity. Their historical perspective lends credibility to the technology’s validation and translation. Even if the assets are relatively new, a thorough understanding of their context, history, and potential demonstrates leadership’s grasp of the technology’s capabilities and future.
The CEO
Startups often feature CEOs with diverse backgrounds, and experience matters. I’m particularly drawn to those who previously held mid-level executive roles in large drug or device companies, where they successfully managed product launches—especially blockbusters. These CEOs bring practical, been-there-done-that expertise, which they now channel into their startups.
Equally important is the CEO’s ability to listen, adapt, and apply feedback. Coachability and a willingness to learn quickly are essential traits for navigating the challenges of the startup journey.
The Management Team
A diverse and complementary management team strengthens a company’s foundation. The best CEOs build teams with varied expertise that enhances their leadership and covers all critical areas. Teams with corporate experience understand when to hold steady, pivot, or fold, and this insight positions them for success. A team with a proven track record of success and the ambition to transition their startup to the next level will attract attention and generate momentum.
Milestones and Data
A well-structured plan with realistic milestones and compelling, validating data is essential. This demonstrates that the product is progressing in the right direction. Credibility is earned when milestones are achieved and data is presented clearly and honestly. Conversely, a lack of coordination erodes trust and opportunities for partnerships. Investors and partners value data-driven, achievable goals that reinforce confidence in the company’s trajectory.
Entrepreneurial Agency
Entrepreneurial agency is hard to define, but it’s unmistakable when you see it. It’s the confidence, passion, professionalism, and determination that propel a company forward. This “mojo” inspires trust and sets companies apart.
While I admire seasoned CEOs from large enterprises who bring their expertise to startups, I also value the energy of dynamic founders driven by their passion to address unmet medical needs.
Drumroll, please: My Companies to Watch in 2025:
Eysz (California, US)

Eysz is transforming brain health by bringing objective biomarkers, traditionally limited to specialists, directly to primary care physicians. Our first product, the Eysz HV Recorder, enables pediatricians to detect epilepsy in children through smartphone-based facial biometric analysis, reducing neurology workup wait times from 9 months to just 2 weeks. This innovation boosts primary care revenue and allows specialists to focus on the most critical cases. With a $10 billion market in cognitive and mood disorder screening and monitoring, Eysz offers a game-changing solution that meets key clinical and financial needs.

EYWA Biotech is pioneering the production of sustainable psychedelic compounds through synthetic biology and genetic engineering. As the first GMP-certified platform of its kind in Latin America, we focus on developing high-quality APIs and formulations for the treatment of mental health disorders such as depression, anxiety, and PTSD. Our innovative approach not only ensures reduced environmental impact but also delivers cost-effective solutions with rapid therapeutic action. With a commitment to advancing mental health treatment, EYWA is at the forefront of the global movement towards next-generation psychiatric care.

Gate2Brain is a biotech company focused on the development of therapeutics that efficiently cross biological barriers such as the blood-brain barrier, using a radically innovative peptide-based patented technology platform.
G2B-002, the first therapeutic proof of concept of our technology platform, is aimed mainly at the treatment of rare pediatric solid tumors.

We are a company formed by the union of nonconformists. On the one hand, scientists who seek to discover who Brazilians really are and, on the other hand, entrepreneurs and investors who understand the value of this discovery for the country.
While other countries in the world are advancing in the sequencing of the DNA of their population, Brazil is still behind. Traditional paths to the evolution of science do not advance. And we are the ones who lose out, because important traits that may be hidden in our genome are no longer identified, putting Brazilians outside the so-called precision medicine.
Gen-t was created to be a platform capable of including Brazil on the map of genomic research. Understanding our origins is the only path to full development. Knowing our identity, we will be able to develop more effective medicines and treatments for our diseases and verify that Brazil is, in fact, one of the countries with the greatest genetic diversity in the world.

GuideAI Health was founded by clinicians with a mission to improve early detection and diagnosis of chronic vascular disease. As interventional radiologists, we’ve witnessed firsthand failures to identify life-threatening disease, which is why we’ve designed advanced AI models to address this critical gap. Our AI-powered platform is the first solution targeting peripheral vascular disease (PVD), which affects 230 million worldwide. By detecting subtle disease markers often missed by the human eye, we enable earlier and more effective diagnosis and treatment to save lives, limbs, and healthcare costs.
IN8bio (Alabama, US)

IN8bio was founded with the mission of developing next-generation cellular therapies for treating cancer. Dr. Lawrence Lamb, one the Company’s co-founders, is a leader in the field of gamma-delta T cells. During his postdoctoral fellowship in transplant medicine, he was the first to describe homeostatic reconstitution of gamma-delta T cells in patients who receive alpha-beta T cell depleted bone marrow grafts. Dr. Lamb also identified an association between gamma-delta T cell levels and disease-free survival in allogeneic bone marrow transplantation. This work formed the foundation of IN8bio’s focus on delivering gamma-delta therapeutics for the treatment of cancer. The Company is developing allogeneic, autologous, iPSC and genetically modified gamma-delta T cell therapies. Dr. Lamb’s research has formed the basis for the company’s Phase 1 and Phase 2 clinical trials, two in newly-diagnosed Glioblastoma and one in leukemia and lymphoma patients undergoing hematopoietic bone marrow transplantation.
Serenatis (England)

Serenatis is developing three novel small-molecule drugs, each with a unique mechanism of action targeting different pathways implicated in OCD, including the glutamate, dopamine, and mu[1]opioid systems. These innovative therapies aim to address the root causes of OCD and offer new hope for patients through precision medicine.
Serenatis is positioned to revolutionise OCD treatment, opening a multi-billion market.

Servatus Ltd is a global leading therapeutics company with first-in-class clinical results for the safe and effective treatment of rheumatoid arthritis using live biotherapeutics. Servatus has built an efficient, integrated drug development pipeline founded upon a pragmatic, results driven approach to R&D. This approach has enabled Servatus to successfully complete a Phase 2a trial in RA, as well as IBS-C (Phase 1) and Insomnia (Phase 2a). Our efficacy results, in combination with an excellent safety profile, mean we are well positioned to access large patient populations with unmet needs, becoming one of the first companies to realize the enormous potential of microbial biotherapeutics.
Value and Trust Co., Ltd. (Korea)

Value and Trust Co., Ltd.(hereafter VNTC) develops solutions to maintain the correct shape of the spine. Our main product is spine correction brace, Spinamic, but we also develop advanced diagnostic and prescription system to help produce more sophisticated devices. Our focus at the moment is on teenage patients with scoliosis(AIS) and we aim to ensure that children with scoliosis maintain the correct shape of spine without surgery.
Our product, Spinamic, is a fabric type scoliosis brace, which is easy to wear and washable, thus more hygienic. Through our brace, we can treat AIS(adolescent idiopathic scoliosis) patients the most effective way with minimized side effects that existing rigid braces have failed to deal with. Our focus is to improve the quality of life of the scoliosis patients without side effects, especially depression. We are now preparing to develop and apply our product and system to various vertebral deformities.
For our goal of improving patients’ quality of life, VNTC works with many spinal experts including spinal mechanic engineers, doctors, product designers, and textile experts.

Wellumio, a venture-backed startup, is transforming acute stroke care with its Axana™ imaging solution. Powered by Pulsed-Gradient Free Imaging—a groundbreaking, non-radioactive magnetic resonance technology—Axana™ brings acute brain scans directly to the point of care, opening new frontiers in imaging applications. Engineered to bypass traditional radiology infrastructure, this innovation breaks down conventional MRI access barriers, delivering portable brain imaging within minutes at the bedside. After demonstrating efficacy in preclinical trials and advancing to human clinical trials, we are now working to bring this transformative solution to emergency clinical settings where every second counts.


 Rhythm Biotherapeutics is a pioneering biotech company addressing a critical healthcare challenge with a unique approach. In this interview, Founder and CEO Darryl Davis shares insights into the company’s mission, recent milestones, and future goals.
Rhythm Biotherapeutics is a pioneering biotech company addressing a critical healthcare challenge with a unique approach. In this interview, Founder and CEO Darryl Davis shares insights into the company’s mission, recent milestones, and future goals.



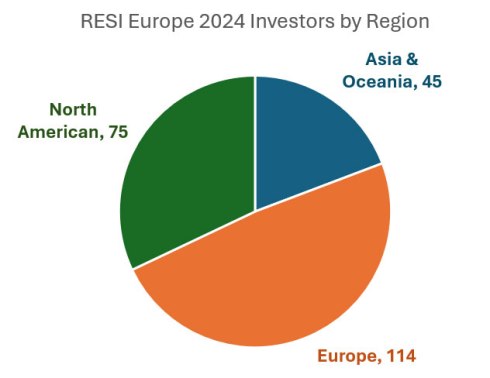
 On April 1st, 2025, Life Science Nation (LSN) will host its 2nd Annual European
On April 1st, 2025, Life Science Nation (LSN) will host its 2nd Annual European