Interview with Sally Stephenson, Founder, Kimaritec Pty Ltd By Caitlin Dolegowski, Marketing Manager, LSN
About the Company:
Caitlin Dolegowski (CD): Can you tell us about Kimaritec PTY LTD and the problem your technology addresses?
Sally Stephenson (SS): Sometime in their life, 1 in 2 men and 1 in 3 women will be told they have cancer. The only treatment that is truly curative is surgery and then, only if the tumour is removed completely, because disease that has started to spread or metastasise is difficult to treat and resistance to current therapies invariably develops. We desperately need new therapies to help people whose therapy has stopped working and change cancer from being an acute disease that will kill people, into a chronic disease that can be treated so people continue living to old age.
Preferably, this would be with limited or at least manageable side effects, so they also have a great quality of life during treatment.
Kimaritec Pty Ltd is an early-stage start-up company from Queensland University of Technology in Brisbane, Australia. Kimaritec started officially in 2020 to explore the commercial potential of research performed by Dr Mohanan Maharaj during his PhD work supervised by Associate Professor Sally Stephenson, a tenured teaching and research academic at QUT. Medicinal chemist A/Prof Wim Meutermans joined the team as COO and contributes significant expertise in drug development.
Kimaritec finds small molecules that cause the cancer cells’ own protein recycling system to remove specific proteins it needs and causes cancer cell death. This is an approach to cause targeted protein degradation.
The way we do this is by blocking specific SUMOylation events. SUMOylation is a post-translational modification, something that happens to a protein after it is made, and one of the things that SUMOylation does is to increase the stability of a protein – keeping it in the cancer cell and allowing it to do its cancer promoting job for longer. SUMOylation is increased in cells that are stressed, and tumour cells are stressed. They are growing without control, pressing on each other, fighting for nutrients and oxygen and they have trouble getting rid of waste. So SUMOylation is increased in cancer cells and a lot of proteins that are not normally SUMOylated now are. Kimaritec’s idea is that if we can identify proteins that the cancer cell specifically needs to SUMOylate and we stop the modification from happening, then we have a new approach to developing useful anti-cancer therapies.
CD: What inspired you to start your journey in this field, and what sets your company apart from others in the industry?
SS: Cancer is one of the worst health challenges that many people will face. Most of us know someone who has had cancer and have seen how devastating this is. Personally, I have had 4 melanomas removed, the worst one before I turned 50, which is young for a disease where the biggest risk factor is getting old. But melanoma is in my family, and I am likely to have many more. In some respects, I am lucky though, because melanoma is on the skin and visible if you know what to look for. But many of our solid tumours develop inside the body and it is difficult to detect them before they become problematic and before they have started to spread. We have made great progress in improving screening for prostate cancer, breast cancer and colon cancer, and people are realizing the importance of regular health checks, but we need to do better.
There is a lot of interest and investment in targeted protein degraders, particularly the PROTACs and molecular glues. They have a different way of causing targeted protein degradation. Their molecules bring a target protein and an enzyme complex together to add a molecular tag called ubiquitin to the target protein and this marks it for degradation by the cell. The PROTAC and glue molecules are a little challenging to work with, but they show that targeted protein degradation works. The Kimaritec approach to targeted protein degradation, by targeting cancer cell-specific SUMOylation events, is a little bit different and new. To our knowledge no one else is doing this in diseases like cancer yet. Also, blocking protein SUMOylation opens up a whole new set of potential targets, including ones that are currently not yet drugged and for which we can be first-in-class.
CD: What milestones has your company achieved recently, and what are your immediate goals for 2025?
SS: We have proven that we can identify small molecules that can specifically block a SUMOylation event on a target protein and stop cancer cells from growing for two cancer proteins.
Our commercial target is a currently-undrugged transcription factor that is required for tumour initiation and progression and a great target for a degradation approach. We have a hit molecule that works in tumour cells growing in a dish – it blocks SUMOylation and stops the transcription factor from moving into the nucleus, and importantly, causes cancer cell death. Our molecule does exactly what we hoped it would do. What we have to do now is the hit-to-lead chemistry required to make this more drug-like and we are looking for people who want to help us do this.
Fundraising & RESI Experience:
CD: How has participating in the Innovator’s Pitch Challenge at RESI JPM impacted your fundraising efforts? Did you receive any valuable feedback or connections from investors?
SS: Kimaritec has been in stealth for the first 4 years while we developed our platform and identified our first targets and molecules. This has been a challenging time for us as an academic research team because we haven’t been able to publish our work and for that reason, we have been passed over many times for Australian grant funding opportunities where track record is mostly measured by publications. The Innovator’s Pitch Challenge at RESI JPM was one of the first opportunities we have really taken to share our ideas with the world and start generating interest in SUMOylation inhibitors. Winning the Innovator’s Pitch Challenge is encouraging and confirms that we do have an innovative idea that is worth pursuing. We have identified a couple of leads and have conversations scheduled for the next couple of weeks. We are hopeful that the right investment for the next stage of Kimaritec is in our near future.
CD: What was your experience pitching to a panel of coordinated investors during the challenge, and how did it help refine your story?
SS: Kimaritec has a cool story, and I enjoy telling people about what we do. I appreciated the great questions, and the interest panel members showed in the science and its potential. Having only 6 minutes to pitch makes you really focus on the points that matter.
Entrepreneurial Education Program:
CD: How did the Entrepreneurial Education program, sponsored by the Brisbane Economic Development Agency, prepare you for RESI JPM? Are there specific takeaways you’d recommend to other entrepreneurs?
SS: The program was great. In particular, learning how to put together a cohesive collection of documents – the one page datasheet, an executive summary, and the pitch deck, then getting feedback on them was very useful. The focus on clarity and continuity in the message in each document was highlighted and the different ways of communicating this information was interesting.
An opportunity to pitch to the “Shark Tank” panel the day before the IPC was great too, and the written feedback did help me see where my message was not as clear as I was hoping, but all I had to do was add a couple of sentences in a couple of places, which I think really improved it.
I would definitely recommend other entrepreneurs, particularly academic scientists like me, take this course. It will help you understand some of the language of business development. Scientists and BD experts need to work together to move companies forward and the more you each learn about the other’s world, the better. I was never taught this in my science degree and to be honest, I wish I had done it sooner. Perhaps I would not have made as many mistakes?
The other benefit was meeting other scientists, bioinnovators and entrepreneurs in Brisbane and surrounds. There are many inspiring and amazing individuals and teams, and I am lucky to have been part of the 2024 cohort.
Closing:
CD: What advice would you offer to other early-stage companies considering participating in RESI or the Innovator’s Pitch Challenge?
SS: Do it. And be an active participant. You will learn a lot. And you will move your company in the right direction.



 On January 23rd, the Trump administration suspended research-grant reviews, scientist travel, external communications, and training at the NIH, causing concern in the worldwide health research community. This included a temporary freeze on 80% of the NIH’s $47-billion budget, much of which goes to funding early-stage research (the NIH awards more than 60,000 grants every year, supporting at least 300,000 researchers).
On January 23rd, the Trump administration suspended research-grant reviews, scientist travel, external communications, and training at the NIH, causing concern in the worldwide health research community. This included a temporary freeze on 80% of the NIH’s $47-billion budget, much of which goes to funding early-stage research (the NIH awards more than 60,000 grants every year, supporting at least 300,000 researchers).


 In this interview, we spoke with Rob Fraser, CSO, President, and Co-founder of Molecular You, who secured 2nd place in the Innovator’s Pitch Challenge at RESI JPM this past January. Rob shares insights into the company’s journey, their fundraising efforts, and their experience pitching to investors at the conference.
In this interview, we spoke with Rob Fraser, CSO, President, and Co-founder of Molecular You, who secured 2nd place in the Innovator’s Pitch Challenge at RESI JPM this past January. Rob shares insights into the company’s journey, their fundraising efforts, and their experience pitching to investors at the conference.



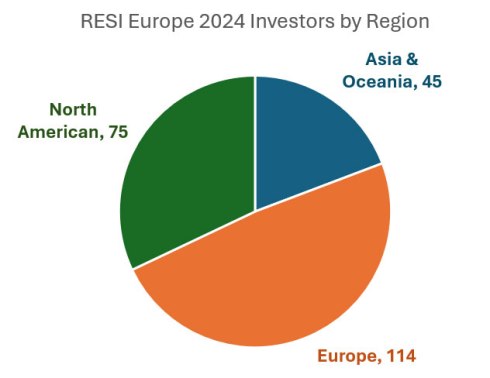
 On April 1st, 2025, Life Science Nation (LSN) will host its 2nd Annual European
On April 1st, 2025, Life Science Nation (LSN) will host its 2nd Annual European