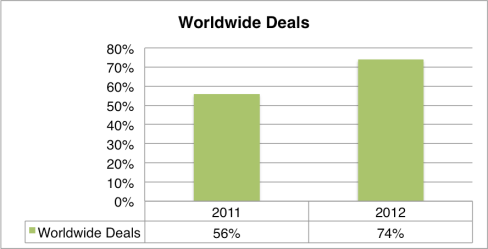
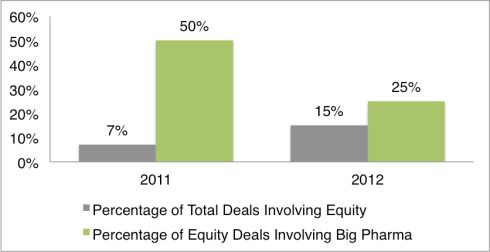
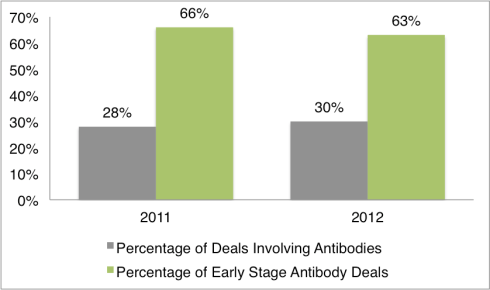
By Tom Crosby, Marketing Manager, LSN
A recent study found that among most small-medium sized businesses, approximately 15% of marketing budgets are allocated towards email marketing (by far the largest single line item). By comparison, SEO and social media budgets came in at 8% a piece, and the confidence levels in the effectiveness of each were dramatically lower than they were for email marketing. This may sound surprising, considering the hype that surrounds social media outlets, but the data clearly shows that new and flashy doesn’t necessarily mean effective. CROs marketing their services should be mindful of how to effectively approach this tool.
In the current state of CRO service marketing inbound leads are few and far between, and small & emerging biotech opportunities are hard to map out. In this environment there is no easier and more efficient outbound attempt that a marketing team can make than email. This is reflected in the findings of the study. Personally, I see the positive effects of our newsletter campaign every day at LSN – most notably is that the few inbound connections that we are able to establish often come directly from the newsletter.
Email marketing is definitely a sales tool. However, there is a right way and a wrong way to go about email marketing. Many marketing professionals make the mistake of believing that the wider your audience is, the better your chances are of making that connection. We’ve all seen the cold emails coming into our Outlook inboxes every morning from marketers at nameless list providers. However, the reality is that a clean and narrow list of good targets is infinitely better than a large, clunky list of cold contacts.
The key to an effective campaign is targeted, interesting content that is being sent to interested readership on a regular basis. You want to create a dialogue with customers so they feel engaged, rather than throwing things against a wall and seeing what sticks. Direct, sales-driven “pseudo-articles” will only cheapen your brand, and will likely put your domain name on the spam list. However, if your prospects feel engaged, you can bring the business to you by solidifying your firm’s position in the marketplace as a leader with niche expertise. In other words, show how your CRO is differentiated from the competition by highlighting the unique advantages you offer. Having created a dialogue with prospects, it will then also be easier for you to adapt as conditions shift.
The aforementioned study showed that one of the main concerns with email marketing was losing subscribers when sending out weekly mailings. In my mind, this is a good sign, because it means that our list is getting stronger, and that we’re not reaching out to people who aren’t a fit for our product. This comes with the added benefit of staying in good favor with the community at large. If somebody doesn’t want to receive emails from us anymore, it’s made quick and easy to just say ‘no thanks.’ This is a simple courtesy that has far-reaching implications in terms of your company’s image – nobody wants to be seen as an annoyance. More importantly, this helps to keep your list fluid and up-to-date, allowing for more targeted campaigns and more effective dialogue.
The key take-away is that email marketing requires planning, strategy, and consistent execution and follow-up. Marketers need to maintain a conversation with their prospect audiences, and make their mailings interesting and easy to read. The goal is to connect your brand with insight and expertise that no one else can offer. When successfully executed, this can be an incredibly powerful tool that enhances the efficacy of the business development team.