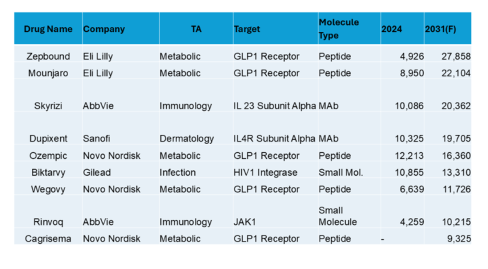
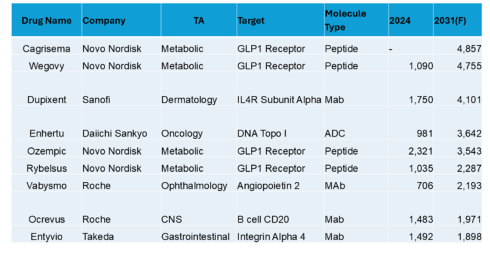
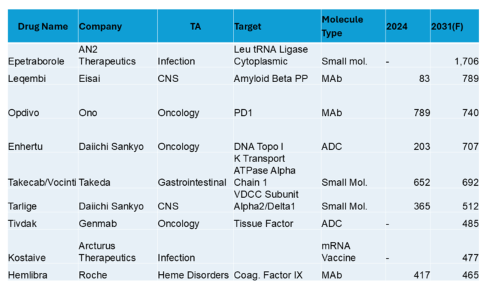
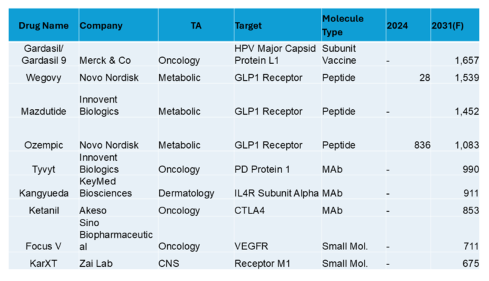
In this interview, Caitlin Dolegowski speaks with Cuong Do, Founder and Chairman of M6P Therapeutics, about the company’s groundbreaking lysosomal targeting platform, its applications in rare disease and oncology, and the experience of pitching at RESI Boston.
 Cuong Do Cuong Do |
 Caitlin Dolegowski Caitlin Dolegowski |
Caitlin Dolegowski (CD): M6P Therapeutics has achieved what was long thought impossible, delivering proteins to lysosomes. Can you explain the significance of this breakthrough?
Cuong Do (DO): An enzyme called GlcNac-1-phosphotransferase (PTase) is responsible for adding mannose 6-phosphate to the surface of lysosomal enzymes. People have tried and failed for decades to increase the expression of M6P, and everybody gave up. Our co-founder Stuart Kornfeld never gave up. He and his post-doc were able to engineer a variant of PTase that turned out to be 20X more effective than PTase itself in adding M6P to lysosomal enzymes. We built upon this breakthrough to create a platform that is able to create enzyme replacement therapies that have very high M6P content. Furthermore, our gene therapies are the only ones that result in M6P-containing enzymes being produced by the transduced cells.
We expanded upon the innovation and created chimeric antibodies that contain M6P as well. This allows these antibodies (after they bind to the targeted antigens) to be brought to lysosomes in virtually all cells in our bodies for degradation. This is a significant advantage over traditional antibodies relying on Fc clearance by only select immune cells.
CD: You have multiple rare pediatric drug designations and two programs nearing the clinic. What are the most exciting upcoming milestones for your pipeline?
DO: We are preparing to start an Investigator Initiated Trial in Australia for our M021 ERT for Pompe Disease in hopes of obtaining early human data demonstrating M021’s superiority over the standard of care.
CD: How does your lysosomal targeting platform extend beyond rare diseases, particularly in oncology with your chimeric PD-L1 and PD-1 antibodies?
DO: We figured out a way to add M6P to any protein, including antibodies. Our chimeric antibodies can be cleared by virtually all cells in the body since virtually all cells have receptors for M6P. This is especially effective for clearing surface antigens from cell surfaces. Our chimeric PD-L1 antibody is able to clear virtually all PD-L1 from the surface of tumor cells and thus activate T-cells and drive T-cell mediated tumor killing. Our chimeric version of Keytruda is able to remove PD-1 from the surface of T-cells and has shown to be more effective in inhibiting tumor growth in vivo than Keytruda itself.
CD: Can you walk us through your IP position and how it supports your growth strategy?
DO: We have invested heavily in IP that has created a portfolio of 9 patent families, 9 issued patents, and ~20 still in prosecution.
CD: Where are you in your fundraising journey, and what types of investors or partners are you looking to engage with?
DO: We have raised ~$40 million in our Seed and A rounds, which we invested to get our programs to where they are today. We are trying to raise a $5 million bridge now in anticipation of a $50+ million Series B next year. In addition to investors, we want to engage with potential partners who might be interested in our molecules.
CD: How did participating in the Innovator’s Pitch Challenge at RESI Boston help advance your business development or investor connections?
DO: We met a few companies who might be interested in partnering on some of our molecules. We’re continuing the conversations.
IPC Applications are now open for the next Innovator’s Pitch Challenge at RESI London 2025 and RESI JPM 2026, with spots filled on a rolling basis.


 Lauren Murdza
Lauren Murdza


















 Eric Sandberg
Eric Sandberg