By Max Braht, Director of Business Development, LSN
The RESI (Redefining Every Stage of Investment) Conference Series, produced by Life Science Nation (LSN), has become a cornerstone event series for the early-stage life science ecosystem. Designed to bring together innovators, investors, and strategic partners, RESI offers a highly curated environment where capital formation, partnership development, and brand visibility intersect.
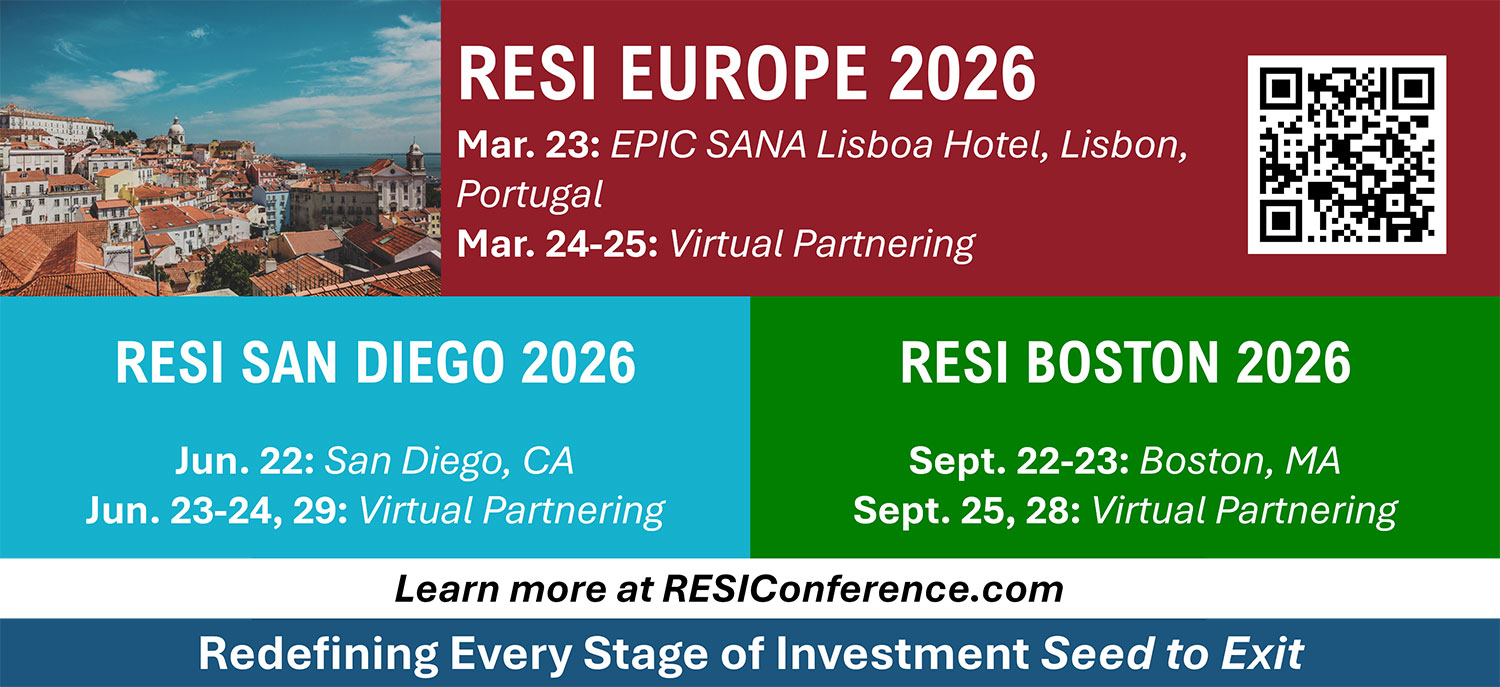
In 2026, the RESI Series continues its global reach through a combination of in-person conferences and structured virtual partnering, offering sponsors year-round exposure and repeated touchpoints with a highly targeted audience.
A Global Series Built for Impact
The 2026 RESI Series includes multiple events across major life science hubs:
- RESI Europe 2026 – Lisbon, March 23 (with virtual partnering March 24–25)
- RESI June at San Diego 2026 – June 22 (with virtual partnering June 23–24, 29)
- RESI Boston 2026 – September 22–23 (with follow-up virtual partnering September 25, 28)
Across these events, RESI convenes companies spanning therapeutics, diagnostics, medical devices, digital health, and enabling technologies, alongside venture capital firms, family offices, strategic investors, and corporate partners. Sponsors benefit from consistent brand presence across the series while engaging with a community focused on early-stage innovation and investment readiness.
Why Organizations Sponsor RESI
RESI sponsorship is structured to go beyond logo placement. Sponsors are integrated into the fabric of the conference experience, with benefits that can include:
- High-visibility branding across pre-event marketing, onsite signage, and digital platforms
- Exhibit opportunities in high-traffic networking areas during in-person events
- Thought-leadership placement, including workshops, moderated sessions, and published articles distributed to LSN’s global audience
- Targeted networking and partnering, supported by RESI’s proprietary matchmaking platform
- Post-event attendee access, enabling meaningful follow-up with investors, founders, and decision-makers
Tiered sponsorship options allow organizations to align their level of involvement with specific business development, visibility, or ecosystem-building goals, while optional add-ons provide further customization.
Who Benefits from Sponsoring RESI
RESI sponsorship is designed to support a wide range of organizations across the life science ecosystem. Sponsors consistently report value not only in exposure, but in the relevance and quality of connections made.
Service Providers
CROs, CDMOs, legal, IP, regulatory, manufacturing, data, and commercialization services (e.g., McDermot Will & Emery, Biometas)
- Direct access to early-stage companies actively building pipelines and seeking partners
- Visibility among founders, executives, and investors at key decision-making stages
- Opportunities to demonstrate expertise through workshops, articles, and curated sessions
Organizations such as Medmarc exemplify the value of sustained participation. Their consistent presence across multiple RESI conferences has helped establish familiarity and trust with early-stage companies, positioning them as a known and credible partner as those companies progress from formation through later stages of growth.
Regional Organizations and Innovation Hubs
Economic development groups, accelerators, incubators, trade organizations, and government-backed initiatives (past sponsors include Brisbane Economic Development Agency (BEDA), Kobe Biomedical Innovation Cluster, (KBIC) and Israel Export Institute (IEI))
- A global platform to showcase regional ecosystems and portfolio companies
- The ability to host demo days, pitch sessions, or dedicated tracks aligned with regional priorities
- Increased international exposure to investors and strategic partners
Investors and Strategic and Corporate Partners
Venture capital, corporate venture, family offices, and strategic partners (past sponsors include, Muscular Dystrophy Association (MDA), Johnson & Johnson Innovation JLABS, and Eli Lilly)
- Targeted visibility among investment- or partnering-ready startups across multiple modalities
- Access to curated partnering and company intelligence through the RESI platform
- Opportunities to participate in panels, pitch sessions, and thought-leadership programming
- Structured environments for scouting, relationship-building, and ecosystem engagement
- Brand alignment with a trusted, innovation-focused conference series
Sponsor Spotlight: How Organizations Activated Their Presence at RESI JPM 2026
While RESI JPM 2026 has already taken place, it provides a strong example of how sponsors can actively engage with the RESI platform — not just through visibility, but through programming and participation that creates tangible value.
One notable example is Kobe Biomedical Innovation Cluster (KBIC), a Gold Sponsor of RESI JPM 2026. KBIC leveraged its sponsorship to host the Kansai Life Sciences Accelerator Program (KLSAP) Demo Day, a dedicated session that highlighted emerging life science companies from its accelerator cohort.
Through this activation, KBIC provided startups with direct access to international investors and strategic partners, while reinforcing its role as a global connector within the life science innovation ecosystem. Rather than serving as a passive sponsor, KBIC used the RESI platform to advance its mission, support portfolio companies, and foster cross-border collaboration.
Similarly, Trillium BIO capitalized on both the high foot traffic generated by its exhibit booth and RESI’s partnering platform to schedule a large number of targeted meetings for its team. By combining in-person visibility with structured partnering, Trillium BIO maximized engagement efficiency and ensured meaningful conversations with potential clients and partners throughout the event.
What Successful Sponsors Do Differently
Examples from RESI JPM illustrate several effective sponsorship strategies that carry forward across the 2026 Series:
They integrate into the program.
Sponsors that host workshops, demo days, or curated sessions create natural engagement opportunities and attract aligned audiences.
They align sponsorship with strategy.
Whether the goal is pipeline development, geographic expansion, or investor visibility, effective sponsors use RESI to support broader organizational objectives.
They prioritize connection over exposure alone.
By leveraging partnering tools, curated meetings, and live engagement opportunities, sponsors maximize the quality of interactions — not just the quantity.
Looking Ahead
As the RESI 2026 Series continues across Europe and the United States, sponsors can build sustained visibility while actively shaping conversations at the forefront of life science innovation. The success of sponsor activations at past events demonstrates that RESI is not simply a conference series but a platform for meaningful engagement, partnership building, and long-term impact within the global life science community.
For more information about sponsorship opportunities across the RESI 2026 Series, contact us at sales@lifesciencenation.com. We look forward to discussing your needs and exploring how RESI can support your goals.




 Laura Vivian
Laura Vivian








